This year marks the 20th anniversary of Her2 breast cancer testing at the Royal Cornwall Hospital. Senior Biomedical Scientist Mary Jones looks back over the past two decades.

Slamon (oncologist and cell biologist) and Ullrich (gene cloner), working together at Genentech California in the 1970s, identified the role of the Her2 gene in the development of breast cancer. Over a decade later Slamon announced details of a novel humanised monoclonal antibody therapy that had been developed to target overexpression of ErbB2, a member the ErbBs family of receptor tyrosine kinases. Clinical trials of trastuzumab (Roche: Herceptin) to treat women with metastatic breast cancer had begun but early trial data showed that not all patients responded to the novel treatment and so a requirement for a predictive laboratory marker(s) came about. It was known that the p185 Her2 protein product on the surface of breast cancer cells correlated with gene amplification and debate in the 2000s was based on the consideration of which test would be most accurate and how this could be standardised in the routine laboratory setting. The HercepTest is a semi-quantitative benchtop immunohistochemistry (IHC) assay, a novel companion diagnostic used in conjunction with fluorescent in-situ hybrisation (FISH) to predict therapy response.
Testing
Royal Cornwall Hospital (RCH) introduced both lab tests in 2004. The HercepTest was purchased as a kit containing buffers, primary antibody, and detection components for undertaking manual IHC. The precision required for this technique made it labour intensive and only possible to run a small batch of slides. The use of Coplin jars in a water bath to maintain 95oC was a technical challenge for antigen retrieval. Accuracy was imperative to optimise epitope exposure and prevent tissue damage. Too hot and a cracked Coplin jar would mean starting the batch again. Initially, the only samples recommended for testing were formalin-fixed paraffin-embedded breast excisions. Improvement in accuracy and further optimisation on breast needle core samples came later when adjuvant treatment became an option. The use of microscopy for interpretation of staining remains unchanged and training was undertaken alongside introduction of the tests.
Optimisation
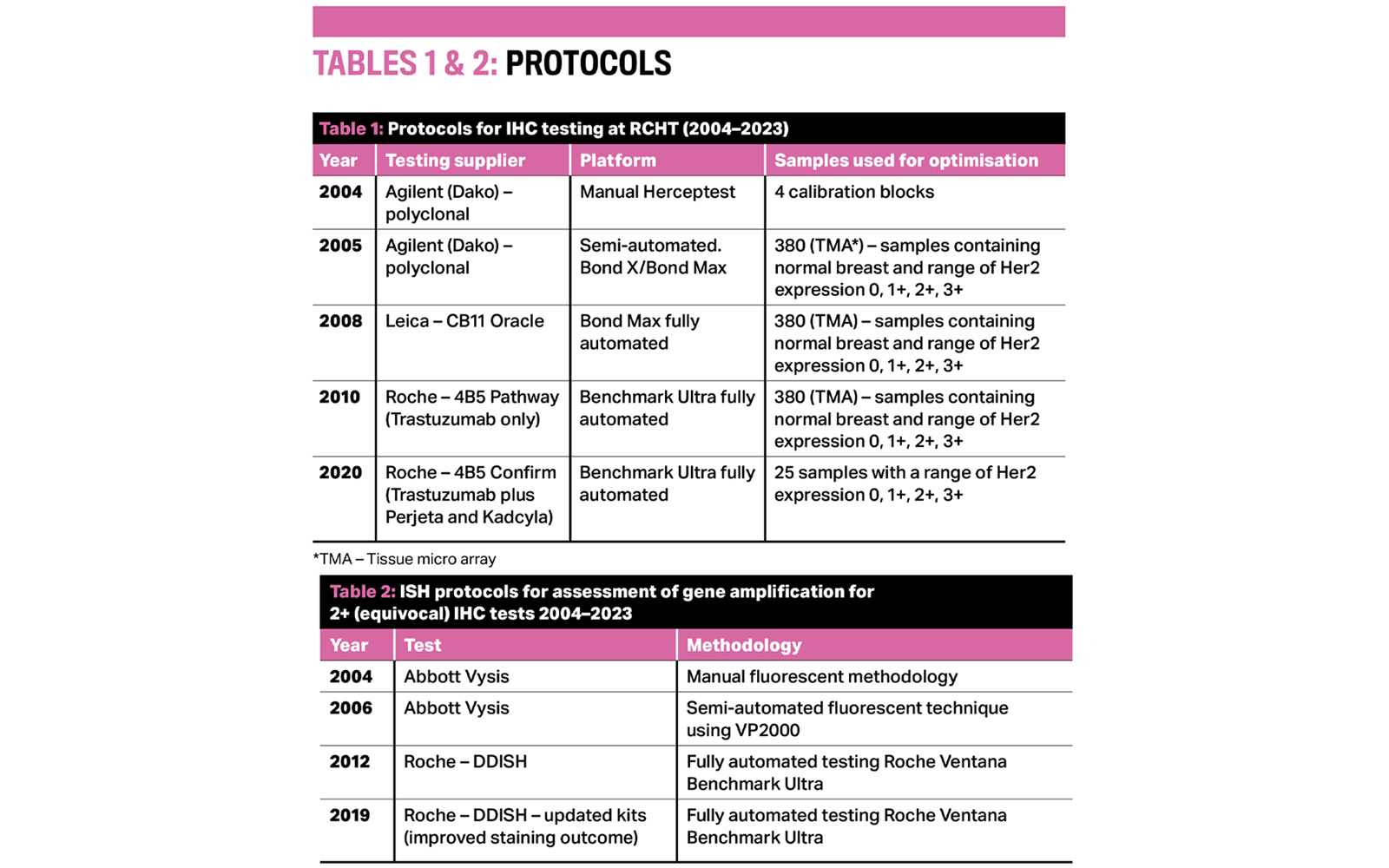
As staining platforms at Royal Cornwall Hospitals NHS Trust (RCHT) were updated, so too the protocols evolved. In 20 years of Her2 testing at RCH, there have been five Her2 IHC testing protocols and three ISH protocols.
Introduction of new testing methods enabled improvement of performance and standardisation. Quality control was carried out per batch until 2021 when same-slide control material was introduced. The commercially purchased cell lines are verified against tissue controls prior to use.
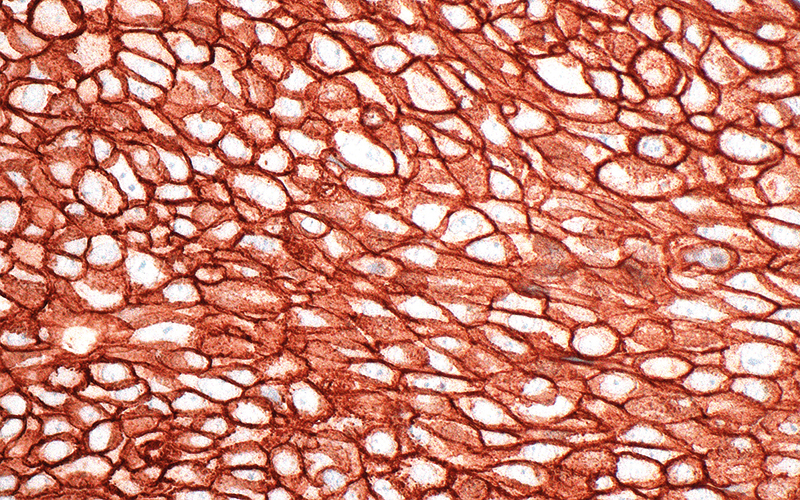
Improvements in automation and standardisation of kits enabled faster turnaround time of results by introduction of daily runs of both IHC and ISH; previously there was just one run of each per week. Both test types, once automated, became part of the routine IHC workload undertaken by biomedical scientists rather than senior and chief biomedical scientists.
Challenges associated with FISH implementation included anomalies in tissue pre-analytics and the specialist skills required in interpretation and imaging of fluorescent probes. Fluorescent microscopy included warming up of the mercury bulb (UV light source), using oil immersion objectives for visualisation of signal, which had propensity to bleach very rapidly, and manipulating z-stacked images to save as a permanent record. Staff training for FISH was more far-reaching and included off-site training by Cancer Research UK, on-site training by pharma experts and sharing experiences with other Her2 testing centres. Some FISH technical issues were eradicated with the change to dual-hapten, dual-colour in situ hybridisation technology. This assay came with different challenges but not fading of signal and so shared reporting became accessible.
Later, NICE was ordered by the UK Health Secretary to fast-track the assessment of predictive testing for all breast cancer patients. Up to this time, testing was requested on an ad-hoc basis and only in the context of advanced metastatic breast cancers for which 25%–30% were expected to be positive for Her2. This was not uniform across the country and the testing had not been rolled out concurrently. The postcode lottery of Herceptin treatment was widely reported by the media and the BBC reported the Cornish success and rollout of Her2 testing and showcased the first patient treated in Cornwall.
Funding
By 2005, primary care trusts in England could fund treatment with Herceptin on early-stage breast cancers. RCHT was awarded the tender for providing the service to the Southwest Peninsular covering five sites. Funding of the service was via by the primary care trust, later superseded by clinical commissioning groups, then integrated care systems, but essentially the funding process as far as the lab functions is largely unchanged in 20 years.
The number of tests increased from 162 in 2004 to >2000 per year IHC in 2023. A key shift in requesting pattern was seen in November 2005 (rollout to primary breast cancer as well as advanced/metastatic breast cancer) and 2008 (retesting of patients with recurrence). There was only a small dip in 2020 during the COVID-19 pandemic.
Automation
Each time a new test was introduced it was optimised against the previous one and with this brings the risk of drift from directly correlating the test against patient outcome. As the number of tests increased, adaptations to the laboratory working practices evolved to ensure accurate results and correlation with UK best practice and registration as a UKAS-accredited test (ISO 15189:2012). Regular audit of techniques, clinical outcome and intra- and inter-observer variation are untaken as part of the audit schedule as well as participation in two EQA schemes.
Electronic result issue – Prior to 2015, distribution of results was a paper copy sent through the post. Later, an electronic email alternative saw a decrease in turnaround time from two weeks to two days for Her2 IHC (not requiring DDISH). Copy holders could also be added to this method of reporting so that results were readily available and, in particular, in time for MDT discussion.
Reporting – In 2004, interpretation and reporting of both Her2 IHC and FISH was undertaken by a breast specialist pathologist. Following publication of the UK Best Methods, a second staff member (biomedical scientist) was added to this process and their responsibility was to record the result at the time of reporting. In 2016, a third staff member (a senior biomedical scientist) was added to this process so that the senior biomedical scientist could be trained to authorise the LIMS record for immediate distribution and quicken access to the result.
As of 2020, a senior biomedical scientist was trained to report Her2 IHC (with a pathologist rostered on for DDISH and where IHC required a second opinion). A training package was established for those who had dual reported approximately 20000 IHC tests and 6500 ISH tests during their careers as biomedical scientists. Keeping up to date with the latest guidelines has been imperative and there have been multiple iterations of UK guidelines within 20 years.
The future
Inevitably improvements in the testing and reporting aspects of Her2 will evolve with advancing research and science.
Of new interest is the Her2-low reporting category, which is a companion diagnostic for trastuzumab deruxtecan (Enhertu). In addition, new reporting technologies that accompany digital slide scanning and the use of artificial intelligence algorithms to aid the reporting of tests will likely become routine practice. Shared LIMS systems will facilitate tracking and access of results to all within the patient care pathway. Potential for new test types, especially within the genomic domain is a possibility and though IHC/ISH are currently the most widely used tests, there is the possibility of them being superseded. The next 20 years will no doubt see more changes to testing provision but with the unified goal of all in the patient pathway to ensure patients have access to the most appropriate treatment based on Her2 expression.
Mary Jones is a Senior Biomedical Scientist working in the Molecular Cell Biology Unit, Histopathology, at Royal Cornwall Hospital.
Image credit | Science Photo Library




