What is Ferguson-Smith disease and how can it be distinguished from cutaneous squamous cell carcinoma? Chartered Scientist Jakub Jawny discusses his research.

Ferguson Smith disease (FSD) is an extremely rare inherited skin condition, clinically and histologically almost indistinguishable from cutaneous squamous cell carcinoma (cSCC). The gene responsible for this autosomal dominant condition was mapped to 9q22-q31 and subsequently mutations in the gene responsible were discovered in transforming growth factor beta receptor 1 (TGFBR-1). Patients typically endure years of multiple enlarging cutaneous tumours, usually on the head and neck (H&N) and on the extremities, which spontaneously resolve within months leaving deep-seated scars and don’t appear to have the capacity for metastasis.
Making a positive diagnosis of an FSD-related tumour is problematic as there is considerable overlap with cSCC and keratoacanthoma (KA). Identification of FSD from biopsy interpretation alone is almost impossible. How can the pathologist approach these cases? Are there any features from the clinical history and histology that may allow us to make a presumptive diagnosis of FSD, which should alert the clinician to take a detailed family history and carry out genetic testing to rule out this rare genetic condition?
A project was developed to examine various factors, including detailed study of clinical and histopathological features of skin tumours in FSD patients with a comparison with cSCC. Retrospective slide review of 40 FSD and 40 cSCC cases was performed. cSCC were divided into cSCC LR (low risk) and cSCC HR (high risk) using defined histological criteria.
Clinical clues
It would appear that over 72% of the cutaneous tumours occur on the H&N, in particular on ears, nose, mouth and lips. A quarter of all tumours appeared on the limbs. The trunk and anogenital region were rarely affected. Tumours on the extremities tended to be much bigger, up to 7cm in diameter in comparison with those on the H&N (2-5cm). The tumours appear as reddish macules, which become larger and ulcerate to form a horny plug which eventually self-resolves to leave a deep scar. The resolving tumours are characterised by areas of immune-mediated inflammation and fibrosis.
Possible precursor lesion
In our study, we potentially identified three phases of evolution (precursor/follicle, invasive and regression) in biopsies of FSD and many of the cases showed multiple phases in one biopsy. It would appear that we could identify a potential precursor lesion where we observed follicular plugging and epidermal expansion in the overlying and adjacent epidermis close to the invasive tumours (Figure 1).
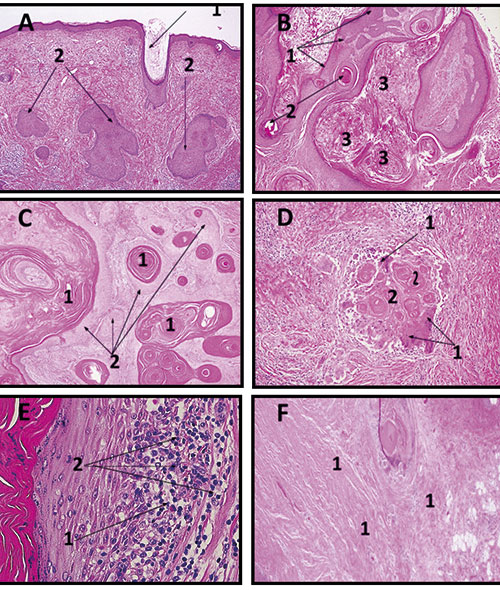
(Left) The stages of FSD tumour progression and evolution.
A is a precursor lesion (Epidermal Stage 1) with mild follicular plugging (1) and epidermal expansion (2).
In B, KA-like lesion (Stage 2), a collar of epithelium is seen (1) with a horn cyst (2)
and the plug, which is filled with keratin (3).
Follicular pattern of invasion (Stage 3) in Figure C with central cystic (1) and with peripheral squamous epithelium (2) are present.
D and E are Inflammatory regression pattern (Stage 4), with macrophage giant cells (2D-1) and with keratin debris (2D-2).
In F there is Fibrotic involution (Stage 5) with collagen deposition (1). HE stain, 200x.
In 15% of FSD cases, an epidermal/follicle phase was found in comparison with 5% and 0% of cSCC HR and cSCC LR, respectively. This is a potentially interesting finding as recently keratoacanthomas and some SCC (follicular/infundibular subtype) are thought to be derived from the hair follicle rather than surface epidermis.
Proliferative phase
Using Scottish Intercollegiate Guidelines Network (SIGN) data set for the reporting of squamous carcinomas, further analysis of cases was performed to assess a presence of histological features, which may confer a HR subtype. Knowing that FSD tended not to metastasise we expected the FSD tumours to show only LR features, but unexpectedly HR features were observed in FSD patients.
Deep invasion beyond the epidermis into subcutaneous fat was found in 32.5 % of FSD cases. Perineural invasion (which is associated with local recurrence in cSCC) was seen 15% and vascular invasion in 5%. In comparison, cSCC HR group had 20%, 20% and 5% of these features present respectively. Statistical analysis also showed the highest median of HR features in cSCC HR of 3.0 following by 1.5 in FSD and 0 in cSCC LR.
Regression
In this stage, invasive elements were infiltrated by an immune cell infiltrate. Here the tumour was under attack from macrophage giant cells with evidence
of cell damage to the squamous tumour cells, which left keratin debris (inflammatory regression). Other areas showed more advanced changes of established regression or fibrotic evolution. Histology showed zones of fibrosis, loss or drop out of tumour cells and immune response to keratin.
Established regression was found in 10% of FSD cases and only in 5% in LR cSCC but not observed in HR cSCC.
Pathology diagnosis
FSD is associated with a high number of lesions (average 20.5 per patient). This should be the very first clue to a potential diagnosis. Also, the study has identical criteria which, although they cannot provide a definite FSD diagnosis, should alert the reporting pathologist to the possibility of an underlying genetic mutation of FSD. These include precursor lesions in the hair follicle, multiple stages of evolution (particularly all in one biopsy) including KA-like areas, well differentiation and areas of inflammatory and established regression. Finally, and quite unexpectedly, the study also showed that HR features such as PNI and VASI do not exclude a diagnosis of FSD, as previously suspected.
Therapeutic model
The project is a preliminary study into the features of FSD and needs to be corroborated by further larger studies, but shows some potential areas of interest. FSD tumours undergo spontaneous involution and regression. The mechanisms involved in these processes may provide extremely important clues to the molecular pathway involved in tumour progression and involution. This knowledge and understanding could help in the discovery of pathways which could be manipulated by targeted therapy in patients with advance and metastatic cSCC. This could potentially be accomplished by stimulating activation of the molecular and immunological pathways in halting and reversing the proliferation of tumour cells.
Jakub Jawny is a Chartered Scientist from Queen Elizabeth University Hospital (QEUH), Glasgow. This article was written with QEUH Consultant Histopathologist Colin Moyes, and Adrian Pierotti, a Senior Lecturer at Glasgow Caledonian University.
Image credit | Science Photo Library




